Once-at-bedtime LUMRYZ is designed differently to treat symptoms of cataplexy or excessive daytime sleepiness (EDS)
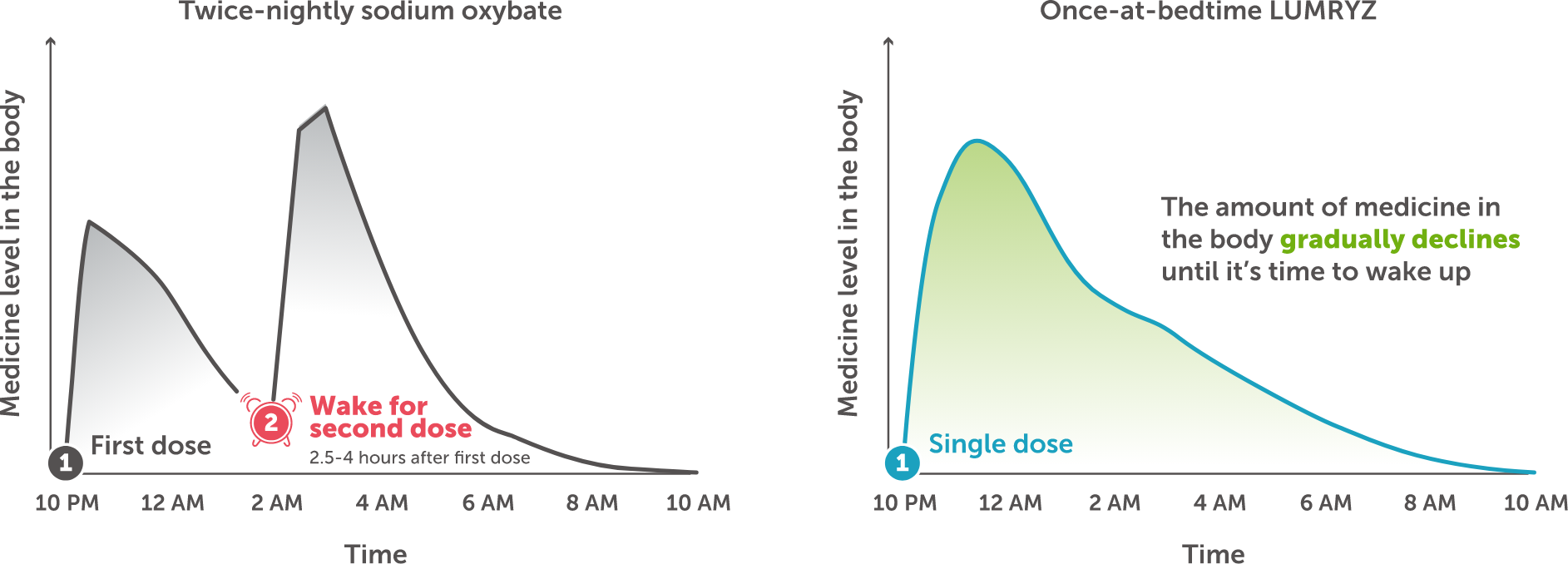
LUMRYZ continues working throughout the course of your sleep
The FDA found once-at-bedtime dosing to be a major contribution to patient care
“Once-at-bedtime dosing provides an opportunity to reduce sleep disruptions and fragmented sleep in a way that is not possible with twice-nightly dosing.
“This is important because the goal of treating sleep disorders is to restore a normal sleep pattern and healthier overall sleep.”
Due to these findings, the FDA has determined LUMRYZ to be clinically superior* to both XYREM® (sodium oxybate) and XYWAV® (calcium, magnesium, potassium, and sodium oxybates).
*Based on a determination of Orphan Drug Exclusivity by the FDA Office of Orphan Products Development between LUMRYZ and XYREM or XYWAV. There are no head-to-head data for LUMRYZ and XYREM or XYWAV.
LUMRYZ contains a unique blend of granules that work in 2 ways:
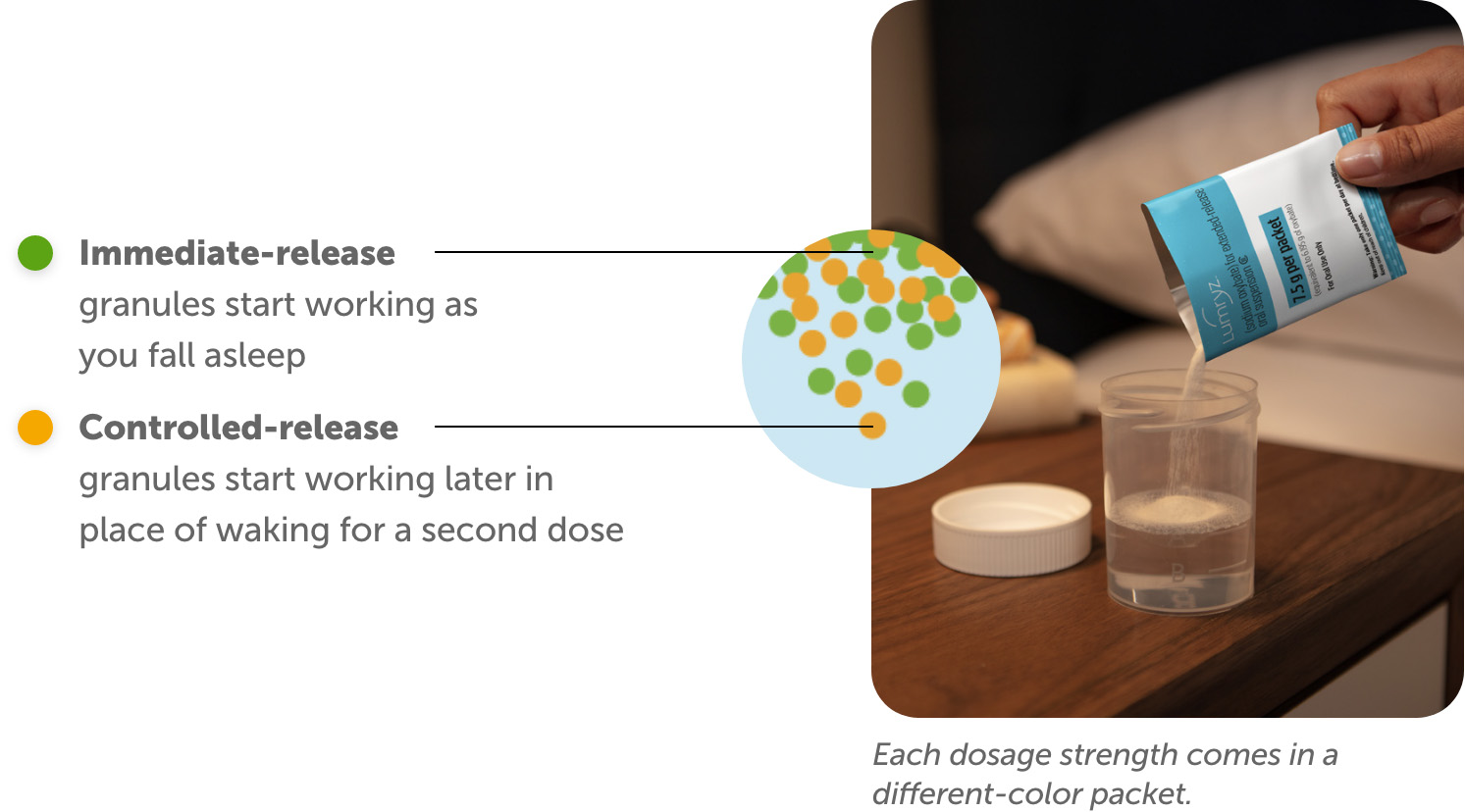
The active ingredient in LUMRYZ is sodium oxybate
Sodium oxybate is strongly recommended by the American Academy of Sleep Medicine (AASM) to treat narcolepsy. This is based on evidence that it reduces cataplexy and EDS.
LUMRYZ is the first and only FDA-approved treatment to help improve symptoms of cataplexy or EDS with its once-at-bedtime dose
“Once-at-bedtime LUMRYZ for me means I can go to sleep thinking about what I’m going to be doing the next day, instead of worrying about missing the second dose of my twice-nightly medication.”
– Katie L., living with narcolepsy and treating with LUMRYZ

Katie was compensated by Avadel Pharmaceuticals
for her time.
Individual results may vary.
The graphs show the amount of medicine in the body at different times during sleep with a twice-nightly oxybate treatment and once-at-bedtime LUMRYZ
Both LUMRYZ and twice-nightly oxybates require that patients not drive for at least 6 hours after taking a dose.
Once-at-bedtime LUMRYZ was proven to help improve daytime symptoms of narcolepsy†
LUMRYZ was studied in a 13-week clinical trial:
Ages 16–72 years
The average age was 31 years old
76% had narcolepsy with cataplexy
Also known as narcolepsy type 1
24% had narcolepsy without cataplexy
Also known as narcolepsy type 2
Participants taking LUMRYZ in this clinical trial experienced‡:
REDUCED EDS
1.5x more wakefulness during the day
LESS CATAPLEXY
57% fewer attacks of cataplexy
OVERALL IMPROVEMENT
73% were rated much or very much improved by clinicians§
In the clinical trial for LUMRYZ, some participants saw significant symptom improvements as early as week 3, while others saw symptom improvements at week 13 after titrating to a higher dose.†
†The LUMRYZ double-blind, placebo-controlled clinical trial included participants with narcolepsy treated with LUMRYZ (n=107). The results measured at week 3 (n=88), week 8 (n=77), and week 13 (n=69), showed daytime symptom improvement of participants on the 6-g, 7.5-g, and 9-g doses of LUMRYZ, respectively. Individual results may vary.
‡As seen in participants taking a 9-g dose of LUMRYZ and compared to baseline results at the start of the trial.
Access a free summary of the LUMRYZ clinical trial.
A once-at-bedtime treatment means no interrupting your sleep for a second dose
In a study§|| collecting the treatment experiences and preferences of participants who switched from a twice-nightly oxybate to LUMRYZ:
69% reported missing their second dose of twice-nightly oxybate either by accident or on purpose
80% said they felt worse symptoms the day after not taking the second dose
94% preferred
once-at-bedtime dosing
93% would recommend LUMRYZ to family or friends with narcolepsy
Results are descriptive and should not be considered clinical evidence.
§At the start of the study, 129 participants shared their experiences taking a twice-nightly oxybate in the last 3 months before switching to LUMRYZ. 98 participants shared their preferred dosing schedule after 3 months on a stable dose of LUMRYZ, and 68 completed a questionnaire at the end of the study to share their perspectives. These are personal preferences and should not be considered clinical evidence.
||This was the secondary objective of the study. The primary objective was to evaluate the long-term safety of LUMRYZ, which found no new safety concerns to report.
With daytime symptom improvement and once-at-bedtime dosing, LUMRYZ can make a night and day difference.
Download the Doctor Discussion Guide to help you get ready for your next appointment.
“Since starting once-at-bedtime LUMRYZ, I feel less sleepy and more awake during the day. I love to get up in the morning and read and write.”
– Wendy B., living with narcolepsy and treating with LUMRYZ

Wendy was compensated by Avadel Pharmaceuticals
for her time.
Individual results may vary.
Side effects of LUMRYZ
The most common side effects reported by participants in the clinical trial were:
Nausea • Dizziness • Bedwetting • Headache • Vomiting
There were no clinically meaningful changes in blood pressure or heart rate.
In pediatric patients, the most common side effects include nausea, bedwetting, vomiting, headache, weight decreased, decreased appetite, dizziness, and sleepwalking.
In the clinical trial, side effects typically occurred when participants started a new dose. Generally, the side effects then declined over time while staying on the same dose.
It’s important to discuss all of your narcolepsy symptoms, goals, and treatment experiences so that you and your healthcare team can work together to find the best treatment fit for you.
LUMRYZ may not be appropriate for some people with narcolepsy. Your healthcare provider can help determine if LUMRYZ is a good fit for you.
